Modeling Wearable and Physiological Data Relationships
Investigating how consumer wearables can capture hormonal and metabolic variations across the menstrual cycle through continuous multimodal sensing and statistical modeling
Unlocking Physiological Insights from Consumer Wearables
This research investigated whether consumer wearables can reliably capture complex physiological variations across the menstrual cycle. Through two complementary studies, I demonstrated that continuous sensing from smartwatches and glucose monitors can reveal meaningful hormonal and metabolic patterns, opening new possibilities for accessible health monitoring.
Two-Phase Research Program
Temperature-Hormone Relationships
Investigating correlations between wrist-worn temperature sensors and daily hormone fluctuations across menstrual cycles
Glucose-Cycle Dynamics
Examining blood glucose variations and their relationship to menstrual phases using continuous glucose monitoring
The Problem
Limited accessibility of hormonal and metabolic health monitoring
Understanding physiological variations across the menstrual cycle typically requires expensive clinical testing and infrequent sampling. Consumer wearables generate continuous data streams, but their potential for capturing meaningful hormonal and metabolic patterns remains largely unexplored in in-the-wild longitudinal studies.
Clinical testing is inaccessible and sparse
Traditional hormone testing requires clinical visits, is expensive, and provides only snapshot measurements rather than continuous tracking of physiological changes throughout menstrual cycles.
Untapped potential of consumer devices
Modern wearables continuously collect temperature, glucose, and activity data, but we lack systematic understanding of how these signals relate to underlying hormonal and metabolic processes across reproductive health cycles.
What I Did
Statistical Modeling
Designed and implemented hierarchical linear mixed-effects models to analyze hormone-temperature and glucose-cycle relationships
Longitudinal Study Design
Coordinated 3-month data collection protocol with multimodal sensing across 49 participants
Signal Processing
Developed preprocessing pipelines for continuous physiological data including filtering, interpolation, and peak detection
Research Approach
Research Goal
Can consumer wearables provide clinically meaningful insights into hormonal and metabolic variations across the menstrual cycle through continuous, accessible monitoring?
- How do wrist-worn temperature measurements correlate with daily hormone fluctuations?
- What are the temporal relationships between hormone peaks and physiological signal changes?
- How does blood glucose vary systematically across menstrual cycle phases?
- What confounding factors must be controlled for in consumer device studies?
Devices: Dexcom G6 CGM,, Fitbit Sense (WST), Mira Plus (LH, E3G hormones)
Duration: 3 months continuous collection per participant
Analysis: Linear mixed-effects models with random slopes/intercepts, quadratic terms, hormone interactions
Outcome: 45 participants, 123 analyzable cycles
Devices: Dexcom G6 CGM, Fitbit Sense, Mira Plus
Duration: 3 months with daily self-report diaries
Analysis: Univariate and multivariate models controlling for step count, food cravings, fatigue, sleep
Outcome: 49 participants, 149 analyzable cycles
Used validated consumer devices (Fitbit Sense, Dexcom G6, Mira Plus) rather than clinical-grade equipment to evaluate real-world accessibility. All devices, in prior literature, showed strong correlations with clinical methods: Fitbit PBT ±0.23°C vs. clinical thermometry, Dexcom 98.7% accuracy vs. lab glucose, Mira 99% ovulation prediction accuracy.
Technical Analysis Framework
Data Preprocessing & Signal Processing
Applied systematic preprocessing with different approaches for temperature vs. glucose data. Temperature signals required noise reduction due to sensor variability, while glucose data needed cycle-aware temporal modeling to capture periodic patterns.
- 5th-order Butterworth low-pass filter with 10% cutoff frequency
- Linear interpolation for temporal standardization (1 sample/day)
- Peak detection via "three-over-six rule" (≥0.2°C elevation for ≥3 days)
- Hormone peak identification: Local maxima >1.3× E3G, >2.2× LH baseline ratios
- Daily median calculation from 5-minute CGM readings (288 samples/day)
- LOESS smoothing for trend visualization across normalized cycles
- Restricted cubic spline analysis for periodic pattern detection
- Quality control: ≥18 hours continuous data per day requirement
LOESS Smoothing & Temporal Visualization
Implemented LOESS (Locally Estimated Scatterplot Smoothing) regression to visualize physiological trends across normalized menstrual cycles. This non-parametric approach revealed biphasic patterns without assuming specific functional forms.
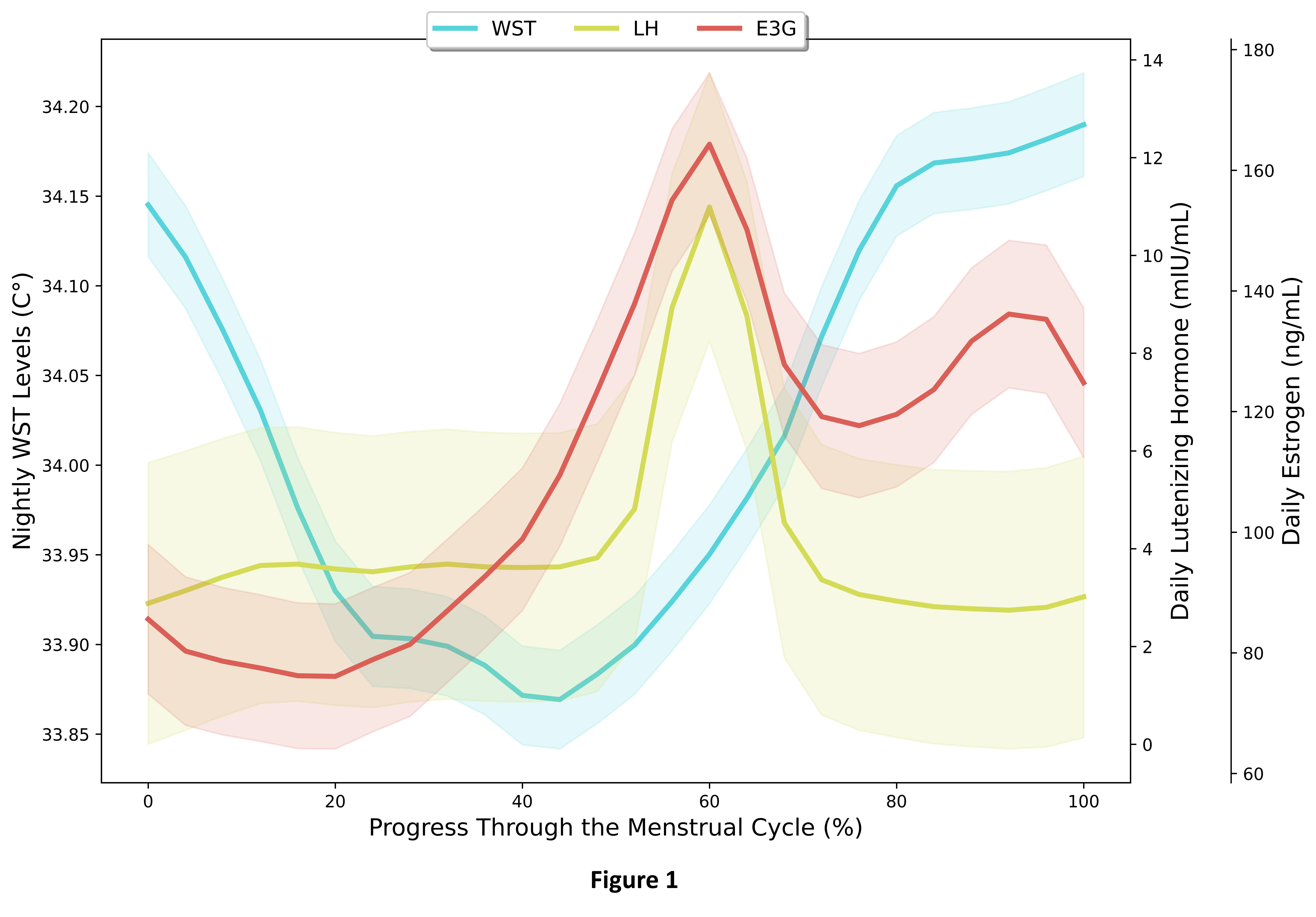
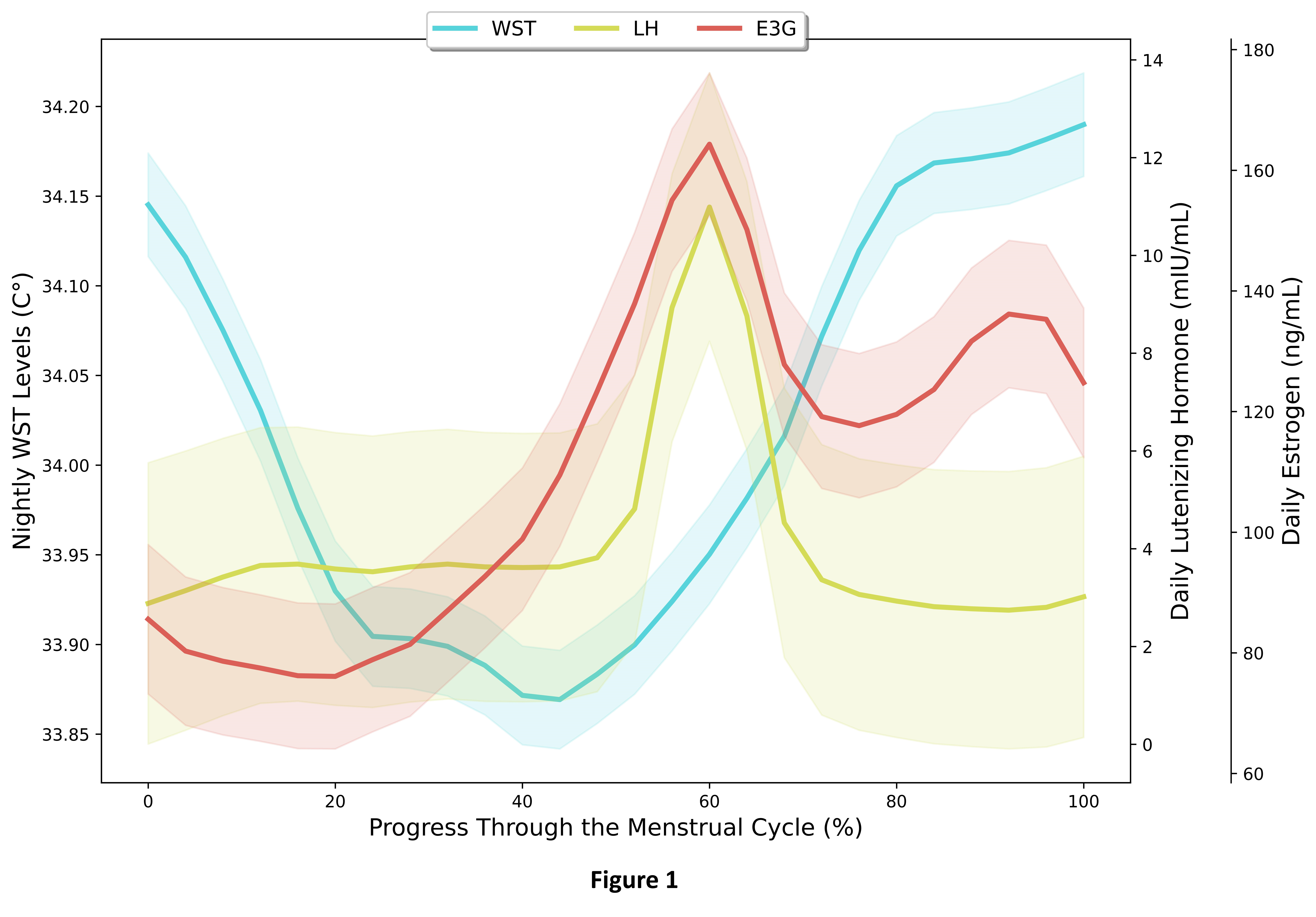
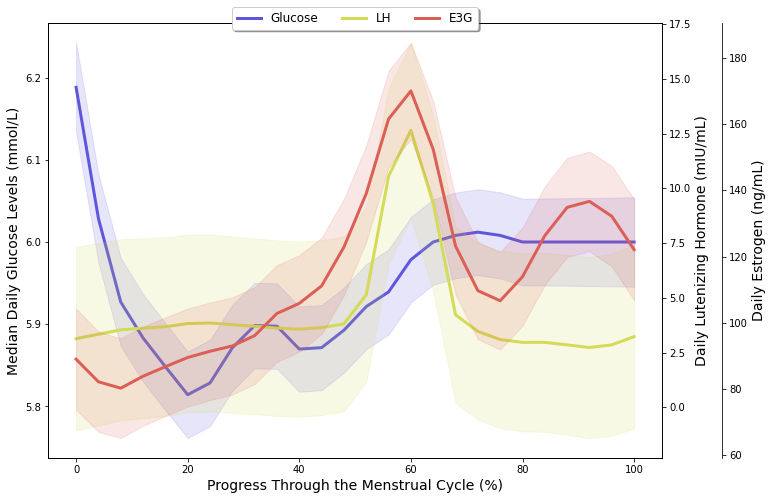
LOESS-smoothed trends revealing biphasic patterns in temperature (Phase 1) and glucose (Phase 2) across normalized menstrual cycles
Periodic Spline Analysis & Cycle Modeling
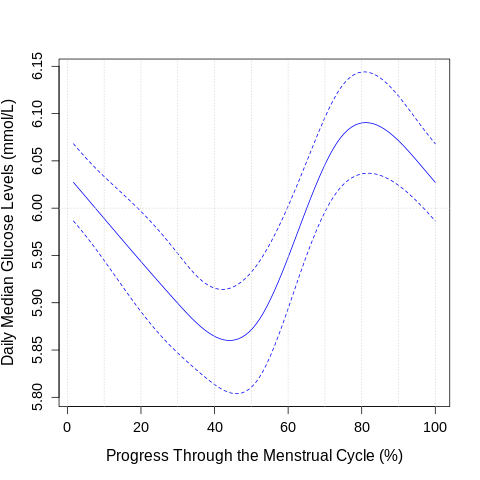
To analyze daily glucose variation across the menstrual cycle, a generalized linear model (GLM) was fitted using restricted cubic splines (RCS). Knots were empirically determined from the data using rcspline.eval(), providing five internal knots that flexibly capture non-linear trends in glucose levels across the normalized cycle timeline.
Custom plotting was applied using the plot_model() function to visualize the spline-predicted relationship between cycle progression (%) and median daily glucose levels (mmol/L). Plot aesthetics were standardized using set_theme() to ensure consistency across figures, with enhanced label visibility and axis clarity.
Hierarchical Mixed-Effects Modeling
Implemented phase-specific linear mixed-effects models with different structures for temperature vs. glucose analyses. Temperature models focused on hormone-physiology relationships, while glucose models emphasized phase-based comparisons. All results of models were reported according to β-coefficients, 95% CI, and the significance of each relationship within models, considering a statistical significance threshold of p<0.05. I applied Holm-Bonferroni adjustments to correct for multiple comparisons.
- Hormone predictors centered by individual participant means
- Quadratic terms to capture non-linear relationships
- Interaction terms between estrogen and luteinizing hormone
- Random slopes for cycle effects within participants
- Phase as categorical predictor (late-follicular reference)
- Linear hormone effects (E3G as continuous predictor)
- Systematic confound testing and multivariate adjustment
- Random intercepts only (no cycle-level random effects)
Confound Control & Multivariate Analysis
Systematically tested potential confounders including demographics, physical activity, stress, sleep quality, and food cravings. Built multivariate models to isolate hormone-physiology relationships from lifestyle factors. Also built models stratified by phases.
Physiological
- Daily step count
- Sleep duration/quality
- Heart rate variability
Behavioral
- Self-reported physical activity
- Food cravings (6-point scale)
- Stress levels
Demographic
- Age, BMI, height, weight
- Cycle length variability
- Previous contraceptive use
Temporal Alignment Analysis
Built peak detection algorithms to identify hormone surges and corresponding physiological responses. Calculated temporal offsets between hormone peaks and temperature changes.
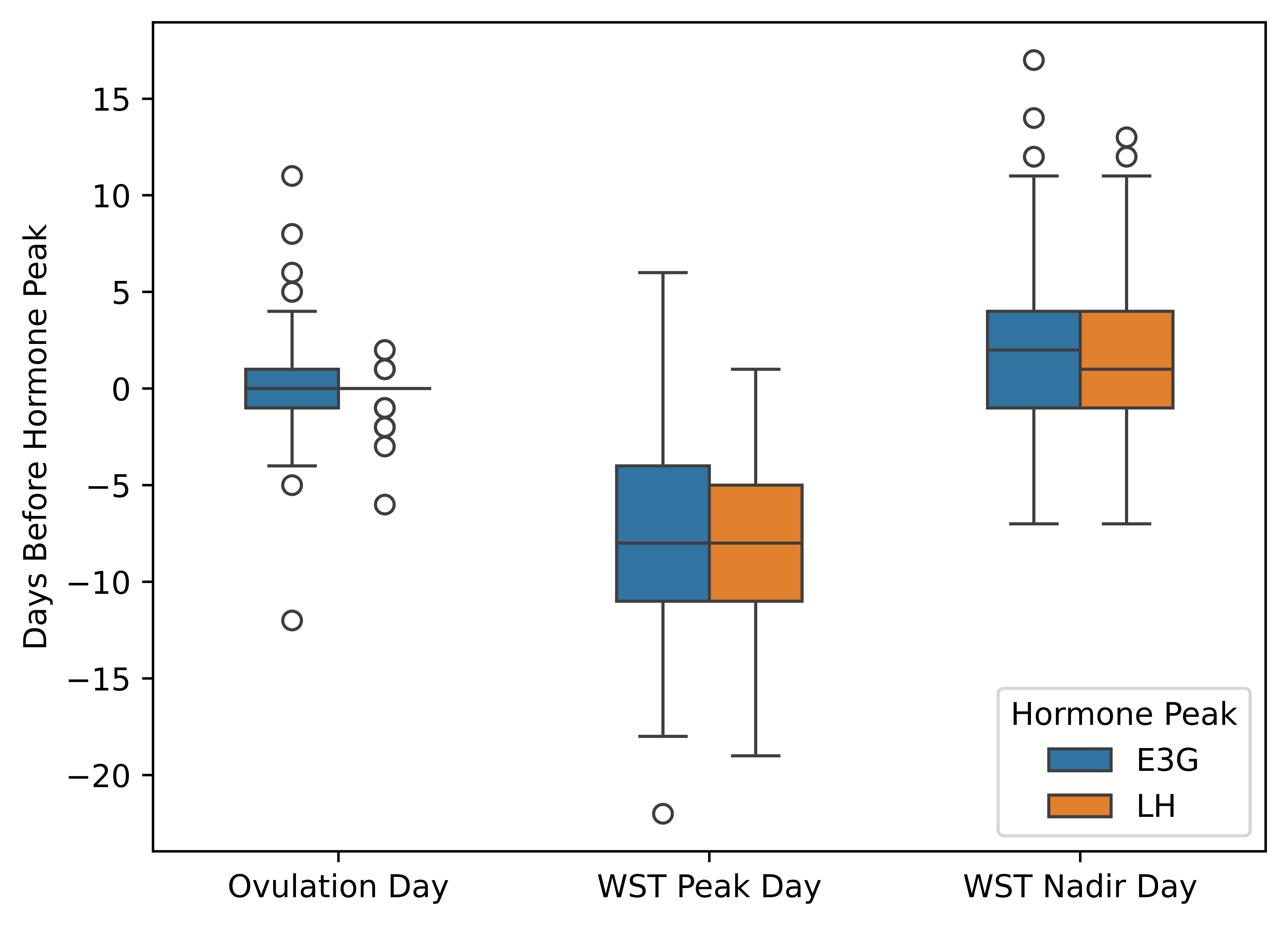
Key Findings
Wrist-worn temperature sensors successfully detected biphasic patterns correlating with hormonal fluctuations:
- Linear LH-temperature relationship: β = -0.002°C per mIU/mL (p < 0.001, CI: [-0.004, -0.0009])
- Phase-specific E3G effects: Higher estrogen → lower WST during ovulation (quadratic term significant)
- Temporal alignment: WST nadirs occurred 2.3±4.5 days before E3G peaks, WST peaks 8.2±4.2 days after
- Robust to confounds: Relationships persisted after controlling for glucose, activity, stress, and sleep quality
Continuous glucose monitoring revealed consistent metabolic patterns synchronized with reproductive cycles:
- Phase-specific elevation: Luteal phase glucose 0.16 mmol/L higher than late-follicular (p < 0.001)
- Ovulation increase: Significant 0.08 mmol/L elevation during fertile window (p < 0.05)
- Estrogen correlation: Higher E3G levels → lower glucose (β = -0.0003, p < 0.01)
- Behavioral interactions: Food cravings amplified glucose elevation during luteal phase
Hierarchical modeling revealed substantial between-person differences requiring individualized baselines:
- Timing variability: Hormone-temperature alignments varied ±4.5 days between individuals
- Cycle length effects: Relationships scaled with individual cycle duration (28.0±8.7 days)
- Random effects significance: Individual intercepts and slopes essential for model validity
Quantitative Results
| Analysis | Significant Relationships | Statistical Significance |
|---|---|---|
| Phase 1: WST-Hormone Models |
|
|
| Phase 2: Glucose-Cycle Models |
|
|
| Confound Control |
|
|
Research Impact
This work demonstrates that consumer wearables can capture clinically meaningful physiological variations. The hierarchical modeling approach and longitudinal design provide a template for evaluating other consumer health technologies in reproductive health contexts.